Submitted by obuadmin on
The blood component label should comply with the relevant national legislation and international agreements. Most EU countries use the international labelling system known as ISBT 128 www.iccbba.org (R5 downloadable from end of page).
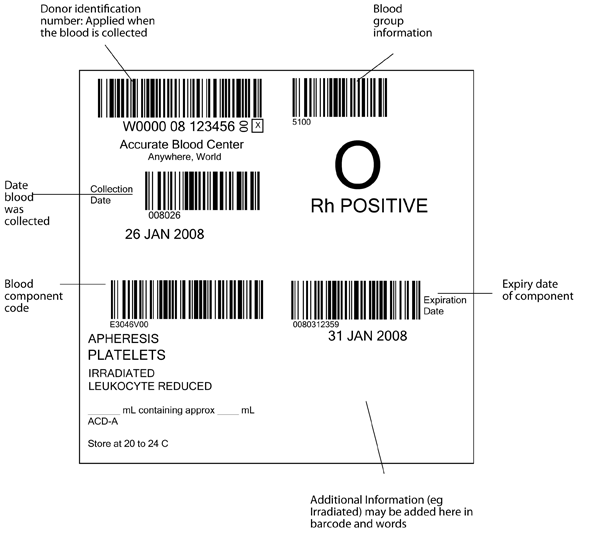
The pack label contains essential information about the blood component, as illustrated in Figure 6.1 and 6.2. The ISBT system requires that the following information be shown in barcode and eye readable form, in the four quadrants of the label.
- Top left: the unique donation number, containing a 5 digit code for the blood establishment, two digits for the year of collection, and a six digit donation number. The blood establishment name and date of the collection must be in eye readable form, (and in figure 6.1 are also shown as a barcode)
- Top right: ABO and RhD blood group
- Lower left: The identification code for the type of blood component (eg red cells, leucocyte depleted in additive solution)
- Lower right: The expiry date of the component. Additional information (eg irradiated) may be added in this quadrant in eye readable and barcode form (see fig 6.2)
Detailed information about barcoding of blood components can be found at www.iccbba.org
Figure 6.1 International ISBT 128 blood component label as specified in the ICCBBA Standard. www.iccbba.org
Directive 2002/98/EC requires that the following information should be shown on the label:
- Official name of the component
- Volume or weight or number of cells in the component (as appropiate)
- Unique numeric or alphanumeric donatior identification
- Name of producing blood establishment
- ABO Group (not required for plasma intended only for fractionation)
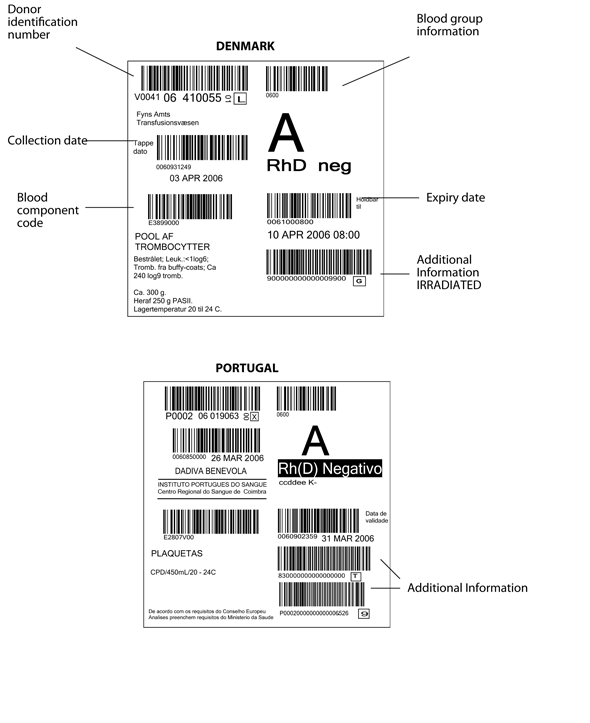
Figure 6.2 Blood component labels from EU countries
Above: Denmark, Below: Portugal
| Attachment | Size |
|---|---|
| 829.2 KB |
