3. Quality systems
Patients’ questions:
One way of introducing the concept of quality management in clinical transfusion is to consider some questions that any patient might ask if they believe that a transfusion may be given.
Here are some examples:
- Do I really need to have a blood transfusion?
- Will it help me?
- Could a transfusion do me harm?
- Will they give me the right blood?
- Will I feel unwell during the transfusion?
- If I start to feel bad during the transfusion will someone come to help me?
- If I need blood in an emergency will they get it to me in time?
- Will someone knowledgeable take the time to explain all this to me?
- Is the hospital staff properly trained to give me the transfusion?
- How do I know that the hospital does these things well?
With these questions, the patient is seeking some evidence that the hospital does a good job in providing blood transfusions.
One way that the hospital can give reassurance is by providing evidence that things are done correctly. This could be information about training, documentation of procedures, or results of checks of performance or comparisons of results between one hospital and others. All these are important parts of a quality system. (11234582)
This Manual provides practical guidance that can help to provide answers to questions of this type, whether they are asked by patients or, in different ways, by quality inspectors, auditors or regulators.
A quality system (QS) for the clinical transfusion process should:
- Provide assurance to patients, the community and clinicians that treatment is safe, effective and efficient, the people who carry out each step of the process know what they are doing, how to do it and why they are doing it
- Provide evidence that tasks are carried out correctly and consistently using the right procedures
- Lead to improvement in quality by providing evidence about performance and by encouraging everyone concerned to learn from both mistakes and successes
Successful introduction of a QS depends on strong management support to make sure that:
- Responsibility for developing and maintaining the QS is clearly assigned
- Sufficient staff, proper working conditions, facilities and training are provided
- An effective programme of evaluation or audit is in place
Why transfusion should be part of a hospital’s wider quality system
Many studies show that patients suffer avoidable harm due to errors and accidents (quality failures) in hospitals. These occur in many aspects of the process of care. For most patients and their clinicians, transfusion is only one element of the whole process of care and transfusion risks are only a small proportion of all the risks to which patients are exposed. For these reasons a quality management system for transfusion should be planned as part of a hospital’s wider quality system. This was a key conclusion of the 2009 Wildbad-Kreuth Symposium.
3.1 Clinical quality assurance
Quality systems have developed largely in relation to manufacturing processes. The same broad principles apply to the clinical setting. However some of the vocabulary, concepts and methods used by quality experts are unfamiliar to many clinicians and also they may not apply directly to the clinical context.
For this reason we have used simple and non-specialist terms where possible. Relevant extracts from the EU directives are shown throughout the text. One relevant definition of clinical quality assurance is:
“Improving performance and preventing problems, through planned and systematic activities including documentation, training and review.”
3.2 Quality system for clinical transfusion
Essential elements include:
Leadership
- Management demonstrates commitment to quality
- Responsibility for quality is clearly assigned
- Resources are available
- There is an effective hospital transfusion committee or equivalent
Standards or specifications
- There are explicit statements of what a product should be or a process should achieve
Documentation
- There are written instructions for doing each job
- There are records to show whether the job has been done correctly
Change control
Changes in procedures are introduced in a controlled fashion and proper records are maintained
Evaluation or Audit
- Performance is independently assessed
Staff Training and Assessment
- Staff are taught what to do and why it is important
- Their knowledge and competence is assessed
Quality Improvement
3.3 Success factors
Professional leadership
A key success factor can be leadership provided by a respected senior clinician who develops an active professional interest in improving transfusion treatment. ‘Clinical Champions’ for good transfusion may emerge from specialties such as anaesthetics/ intensive care, surgery or haematology, where transfusion is frequently utilised. One approach that has been successful is to engage such specialists in collaborative programmes of clinical audit or research on the use of transfusion in their own specialist field.
Effective Hospital Transfusion Committee
An effective and well-led Hospital Transfusion Committee (HTC) or a body with equivalent functions is widely held to be essential for improvement of clinical transfusion practice. The primary aim should be to promote a high standard of care for patients at risk of transfusion (i.e. those who must be transfused, and also those who, with good clinical management, may avoid the need for transfusion). The HTC should have a clear line of accountability to an appropriate post at a senior management level in the institution. The HTC should have the authority to determine hospital policy in relation to blood transfusion and must have an effective means of disseminating it to all relevant staff and to patients where appropriate. (www.transfusionguidelines.org.uk)
Terms of Reference for an HTC
Should include the following:
- Promote the dissemination and the use of national or local guidelines that apply to the clinical transfusion process
- Regularly review and update the hospital’s documentation for blood transfusion
- Carry out audits that evaluate the hospital’s clinical blood transfusion process against the relevant guidelines and benchmark the use of blood components against best practice
- Promote the education and training of clinical, laboratory and support staff involved in the clinical transfusion process
- Report serious adverse reactions and events to the national haemovigilance programme
- Ensure that incidents are analysed and the information is used to help improve practice and prevent a repetition
Membership of HTC
The HTC should include clinicians from specialties in the hospital that utilise transfusion, for example haematology, anaesthetics, intensive care, surgery, or obstetrics, as well as staff from nursing, blood bank and audit or research departments. The committee requires an effective chairperson who has the professional respect of senior medical personnel and can command the attention of hospital management.
Operation of HTC
The HTC should meet regularly, have a formal agenda and keep full records of its decisions. It must have the authority and support to ensure that its decisions are effectively communicated to and followed by staff who contribute to the clinical transfusion process.
Someone who is employed to make things happen
The transfusion committee may make excellent recommendations, but it needs an executive officer, a person who is employed specifically to ensure that the recommendations are converted into actions. Several countries have created a new position for this purpose. The manual uses the term Transfusion Practitioner (TP) (www.betterblood.org.uk) but posts with similar responsibilities have also been given titles such as Transfusion Safety Officer (TSO), Transfusion Nurse Coordinator (TNC) or Haemovigilance Officer (www.transfusionsafety.ca). The TP is concerned with the clinical transfusion process, ”taking quality assurance from the blood bank to the patient”. The transfusion practitioner’s job description would typically specify responsibilities such as these:
- Education and training of nursing and medical staff
- Patient information
- Promote compliance and safety in activities such as specimen collection, administration blood components and products
- Carry out transfusion practice audit
- Investigate and report adverse events and reactions
- Trouble shoot and take preventive and corrective action
- Support development and implementation of transfusion policies and guidelines
In many countries the TP have a background in nursing or the transfusion laboratory; other countries have employed doctors or pharmacists in similar roles. The goal should be for the TP to be part of a wider transfusion team that should develop with the encouragement and motivation of the transfusion committee. In several EU countries, the TP role is now viewed as an essential part of the hospital’s quality improvement programme in transfusion.
3.4 Hospital Transfusion Team
The Departments of Health in the UK have recommended that hospitals have a Hospital Transfusion Team (HTT) to manage the day-to-day business of blood transfusion within the hospital. Membership should include a medical transfusion specialist, the head of the blood bank and the transfusion practitioner.
3.5 Managing the environment
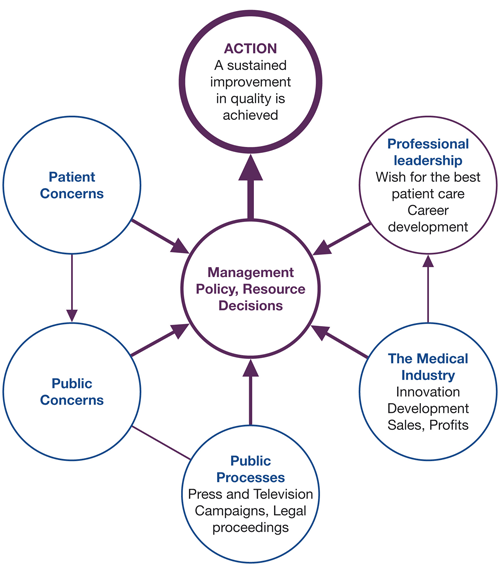
Success in creating change and improvement depends on factors other than the scientific or technical. It is important to be aware of the many influences on the ability to make change happen. Awareness of pressures such as those illustrated in Figure 3.1 can improve the ability to influence decisions and actions. Research also shows the importance of a better understanding of psychological and behavioural factors that underlie the behaviours of health care professionals.
Figure 3.1 Environmental factors affecting quality
3.6 Quality indicators for blood transfusion
Evaluation of the clinical use of blood products is often done by monitoring or surveying clinical practice against objective indicators of performance. This is perhaps better described as benchmarking than audit. Useful indicators of practice (quality or performance indicators) must be easy to collect and quantifiable.
Quality indicators may be used to monitor and evaluate the quality of the therapeutic transfusion process or compliance with clinical guidelines. There are two types of indicators: internal and external.
Internal indicators are used for quality management and improvement of the clinical transfusion process within an institution. They must be relevant for the critical steps in the process and the professionals involved. They must be specific and detailed, easy to sample, educative and effective in stimulating actions for improvement.
External indicators provide information for external control agencies such as health care inspectorate and/or for comparison between hospitals (benchmarking). These have to provide monitoring or signaling information about the quality of the process, measure global aspects such as global outcome and require good validation. According to what they measure three types of indicators can be described:
Structure indicators: How well have I organised the process?
Process indicators: Am I doing well?
Outcome indicators: Do I reach the result required?
Indicators are only one tool for evaluating practice. In some cases audit may provide better information. However, if used in the right way indicators may be an efficient and tool for improving the quality of the therapeutic transfusion process.
3.7 Specific indicators of transfusion practice
The following list is a practical example from the Leiden University Hospital in the Netherlands, where indicators are sampled annually and reviewed by the Hospital Transfusion Committee. This identifies priorities and sets targets for evaluation.
Management of hospital stock
The number of expired products in the stock of the hospital blood bank divided by the total number of blood products in the stock of the hospital blood bank.
Prescription
The number of units of blood components (red cells, platelets and fresh frozen plasma) that are not prescribed according to the known guidelines, divided by the number of prescriptions for blood products (red cells, FFP, platelets) in the same period.
Ordering and wastage
The number of blood components (red cells, platelets and fresh frozen plasma) returned to the hospital blood bank by a department, divided by the total number of blood components supplied by the blood bank service to that department.
The number of blood components that are not transfused divided by the number obtained from the blood establishment.
Request forms
The number of blood product request forms lacking essential data divided by the total number of orders for blood components in the same period.
Patient and blood sample identification
The number of detected discrepancies in ABO and RhD typing of patients due to identification or labeling errors outside the transfusion laboratory divided by the total number of patient samples tested for ABO and RhD type screenings in the same period.
Compatibility testing
The number of detected discrepancies in ABO and RhD screening of patients due to errors in the transfusion laboratory divided by the total number of ABO and RhD type screenings performed in the same period.
Traceability
The number of units for which there is no record in the hospital blood bank or blood establishment of the final destination (transfused to an identified patient, destroyed or returned to the BE) divided by the number of units issued by the HBB or BE.